Common cubic structures
The cubic crystal system is one of the most common (and by far the simplest) class of crystals. As the name itself suggests, all crystal structures of this system have a cube-shaped unit cell with edge length given by the lattice parameter ‘a’.
Here we will discuss the cubic structures with one kind of atom only. The three main common representatives are:
- the simple cubic structure (sc) (also known as primitive cubic)
- the body-centred cubic structure (bcc)
- the face-centred cubic structure (fcc)
In the following sections you can learn more about these structures and make you own 3D paper models. Use your paper models to explore the nature of the structures. Can you answer the questions at the end?
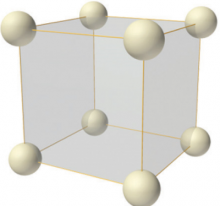
The simple cubic structure (sc)
In the simple cubic structure there is only one lattice point at each corner of the cube-shaped unit cell. They mark the position of either a single atom, or the same group of atoms, known as the motif, which is repeated across the lattice.
The simple cubic structure with only one atom per lattice point is relatively rare in nature, as it is fairly unstable because of its low packing efficiency and low number of nearest neighbour adjoining each atom. Polonium (Po) is reported to crystallize in the simple cubic structure.
Make your own 3D paper model of a simple cubic structure with our cutting instructions, and explore its nature.
Instructions and learning points Template
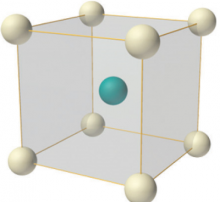
The body-centred cubic structure (bcc)
The body-centred cubic structure is based on the simple cubic structure, but has an additional lattice point at the very centre of the cube. The packing efficiency and the nearest neighbour interaction are slightly higher than for the simple structure, so it is more common in nature. Prominent metallic solids with this structure are Irom (Fe), Chromium (Cr), and Tungsten (W).
Use the following instruction to make your own 3D paper model of a body centered cubic structure.
Instructions and learning points Template
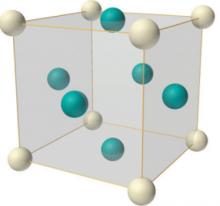
The face-centred cubic structure (fcc)
Another structure based on the simple cubic structure is face-centred cubic. In this crystal structure there are the eight lattice points at each corner, as in the simple cubic, but there are additional lattice point at the centre of each face of the cube. This structure is very common for metallic elements as it maximises the packing efficiency and has the highest number of nearest neighbours, increasing its stability. This is why the fcc structure is sometimes also called the cubic close-packed structure.
Instructions and learning points Template
Questions
-
How many nearest neighbours does each atom have? Take into account that each cubic unit cell is surrounded by several similar unit cells.
-
How many atoms are in each cubic unit cell?
-
What is the distance between nearest neighbours? (Use the length of the cube edge ‘a’ as a scaling parameter.)
-
The packing efficiency describes the ratio of the maximal volume occupied by the atoms and the volume of the cubic unit cell. In your 3D paper model, now assume that the neighbouring atoms are touching each other, then, what will be the packing efficiency for these structures? Compare the result with the values of other crystal structures.


