What is a crystal?
 Crystals are everywhere around you! They are not always obvious, but without them your smartphone and laptop would not work, and you would not get any electricity out of the sockets in your room. You can find crystals in your kitchen as well, for example in form of rock candy and salt.
Crystals are everywhere around you! They are not always obvious, but without them your smartphone and laptop would not work, and you would not get any electricity out of the sockets in your room. You can find crystals in your kitchen as well, for example in form of rock candy and salt.
You can break crystals into small pieces which you can examine under a microscope. Under special high resolution microscopes one can see that crystal consists of many, many atoms. This is common for all solids, but crystals are very special as these atoms are ordered in a pattern that repeats itself, like in a net.
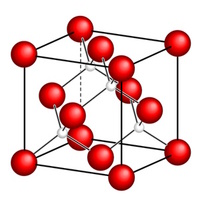 This pattern is what researchers call a crystal lattice. Each node of the crystal lattice represents a lattice point, which marks the position of either a single atom or a particular group of atoms. A small region of the crystal lattice can be used to represent the whole crystal because we know that crystals have a regular, repeating structure. This region is called a unit cell.
This pattern is what researchers call a crystal lattice. Each node of the crystal lattice represents a lattice point, which marks the position of either a single atom or a particular group of atoms. A small region of the crystal lattice can be used to represent the whole crystal because we know that crystals have a regular, repeating structure. This region is called a unit cell.
The meshes of the crystal lattice are called unit cells. By sticking them side-by- side to each other one can rebuild the whole crystal without generating a gap or hole in the crystal. In this way the pattern of atoms of each unit cell is repeated across the lattice.
There exist many different types of crystal structures with different shapes and atom patterns. On the next pages you can see some common crystal structures by building 3D models.
| Back to the Main Menu | Learn about common cubic structures〉


